Serialized inventory tracking is no longer a niche capability-it’s become a core requirement for fulfillment environments where traceability, compliance, and unit-level control are non-negotiable. In high-stakes sectors like medical devices, premium wine, electronics, and luxury goods, serialization is now treated not as a process enhancement, but as operational infrastructure.
What is Serialized Inventory Tracking?
Serialized inventory tracking refers to the practice of assigning a unique serial number to each individual unit of inventory and maintaining that identifier across all warehouse operations and system integrations. Unlike lot or batch tracking, serialization allows every item-down to the single device, bottle, or component-to be traced independently.
But serialization doesn’t end with labeling. In practice, it involves:
- Serial number generation according to business rules or regulatory formats
- Scanning and validation during receiving, putaway, picking, and packing
- Recording serialized transactions in the WMS and synchronizing with ERP, compliance, or POS systems
- Linking serials across packaging hierarchies (unit, case, pallet) for multi-level or nested serialization
- Maintaining a full audit trail for every serialized item across its lifecycle
In short, serialized tracking makes it possible to answer not just “how many,” but “which one, where, when, and what happened to it.”
Why serialization has become mission-critical for fulfillment teams
With rising compliance demands (e.g., FDA UDI, TTB, CPSC), increasingly complex product configurations, and growing pressure to support returns, warranty claims, and post-sale diagnostics-serialization enables fulfillment teams to:
- Trace units from receipt through shipment and beyond
- Authenticate products and detect counterfeits or theft in-transit
- Enforce chain-of-custody policies and regulatory mandates
- Support high-volume operations without sacrificing data integrity
In environments where even a single mislabeled or untracked unit can result in fines, fraud, or reputational damage, serialized inventory tracking software is being leaned on to deliver end-to-end visibility and accountability.
Understanding Serialized Inventory
Serialization, at its core, involves assigning a unique identifier-often a serial number or encoded data string-to each discrete unit of inventory. This identifier is used to track the item across all warehouse activities, system integrations, and supply chain touchpoints. While the concept may seem straightforward, its implementation is anything but-especially when multiple levels of packaging and varying regulatory frameworks are involved.
Levels of Serialization
Inventory can be serialized at one or more of the following levels:
- Unit-Level Serialization: Each individual item receives a unique serial number. This is standard for high-value products like smartphones, laptops, medical implants, or luxury watches. It allows for precise traceability, warranty validation, and fraud prevention at the item level.
- Case-Level Serialization: A serial is assigned to a case containing multiple units. This may be used when unit-level tracking is unnecessary or when secondary traceability (e.g., for replenishment tracking) is sufficient.
- Pallet-Level Serialization: The highest packaging level. Pallet-level serials are used primarily for logistics optimization, automated warehouse handling, and batch-level traceability in large-scale operations.
- Multi-Level or “Nested” Serialization: This involves maintaining traceability across hierarchical relationships-pallet → case → unit. It ensures that a unit’s serial is linked not only to itself but also to the parent case and pallet. This is critical for industries that require cradle-to-grave traceability, component-level integrity, or regulatory auditability.
Real-World Examples
- Wine bottles: Serialized at the unit level (bottle) for authenticity; linked to case and pallet for regulatory auditability (TTB compliance).
- Jewelry pieces: Serialized individually, often with RFID or QR codes, and nested within tamper-evident cases.
- Consumer electronics: Component-level serialization within serialized devices (e.g., motherboards, chips within a laptop); often nested into serialized cartons or pallets for distribution.
When Item-Level Serialization Isn’t Enough
Item-level serialization offers a strong foundation for traceability, and in many operations—especially those with simpler product flows or lower compliance risk—it’s more than sufficient. However, certain industries and product categories require more than just unit-level tracking to meet evolving regulatory, operational, and customer expectations. In these scenarios, single-layer serialization often reveals limitations.
Why Some Industries Need Multi-Level Serialization
- Loss of context in shipment traceability: When only the individual item is serialized, it becomes difficult to trace its association with a larger shipment—such as the case, pallet, or inbound container it was packed in. This lack of hierarchical linkage makes it harder to isolate quality issues, detect tampering, or verify chain of custody.
- Returns and warranty without shipment-level linkage: A returned product might carry a valid serial number, but without knowing the case or pallet it originated from, it’s challenging to perform root-cause analysis or validate warranty claims. This is particularly relevant in high-value goods like premium wine, where provenance and environmental conditions across the entire supply chain matter.
- Regulatory and audit demands: In industries like medical equipment or high-end electronics, regulatory bodies often expect full visibility across packaging hierarchies—not just at the item level. During recalls or audits, being able to quickly pinpoint affected units at the pallet or case level is critical.

Not One-Size-Fits-All
Multi-level serialization—while powerful—comes with added operational costs. It can increase the time required for receiving, inventory counting, and fulfillment tasks. That’s why it’s best suited for use cases where traceability is a non-negotiable requirement due to compliance, risk, or product value—not for every operation.
Real-World Implications
- Recalls: A failure to link units to specific cases or pallets can lead to over-scoped recalls-pulling thousands of unaffected items due to insufficient traceability.
- Returns & Warranty: Serial validation without parent-level context often creates false negatives in warranty systems, leading to manual overrides or customer dissatisfaction.
- Inventory reconciliation inefficiencies and write-offs: In operations without multi-level serialization, mismatches between system records and physical inventory are more common—especially at the case or pallet level. Without a clear parent-child relationship between items and their packaging, cycle counting becomes labor-intensive and error-prone. As a result, businesses often face avoidable write-offs for stock that exists physically but cannot be confidently accounted for in the system.
The Role of Multi-Level Serialization
Multi-level serialization offers a powerful way to extend traceability beyond the item level—by linking each serialized unit to its parent containers (like cases or pallets) and to the overall shipment it was part of. This nested structure enables deeper visibility and control across the fulfillment process.
That said, multi-level serialization is not a one-size-fits-all solution. In many industries, single-item traceability is sufficient and more operationally efficient. But in others—such as premium wines, high-value medical devices, or regulated electronics—a layered serialization model is essential to meet compliance, reduce write-offs, and streamline returns.
When needed, multi-level serialization enables:
- Contextual traceability: Know how, where, and in what configuration each item was packed.
- Packaging integrity checks: Validate shipment or pallet contents during receiving or transit.
- Effortless audit readiness: Regulatory inspections can traverse packaging hierarchies with confidence.
At Hopstack, we support both simple and nested serialization models across all fulfillment workflows—so brands and 3PLs can tailor their traceability strategy to the complexity of their products, industry requirements, and operational cost constraints.
Why Serialized Tracking in WMS is Important?
When serialization is natively supported within a WMS-rather than bolted on through manual processes or disconnected tools-inventory moves with intelligence, accountability, and complete traceability. Each serial number becomes a thread that ties together not just location data, but process integrity, compliance readiness, and customer service capability.
End-to-End Traceability (from Manufacturer to Customer)
Serialized inventory enables granular, event-level tracking of each unit as it moves through the supply chain. A properly integrated WMS captures:
- Who received the item
- Where it was stored
- When it was picked, packed, and shipped
- Which carrier delivered it and to which customer
- Whether it was returned, replaced, or recalled
This traceability extends well beyond warehouse walls, especially when the WMS syncs with ERP, MES, or customer service systems. The result is full lifecycle visibility-not just for internal ops teams, but also for compliance officers, brand protection units, and customer support.
Example:
A manufacturer of orthopedic implants uses Hopstack to track each device from the time it enters the warehouse to the moment it's delivered to a hospital. The WMS logs who received it, where it was stored (temperature-controlled zone, if needed), when it was picked and packed, and which carrier delivered it. If the hospital later reports an issue with the implant, compliance teams can pull the full chain of custody within seconds—satisfying both internal QA and external regulatory demands.
Streamlined Recalls and Returns
When recalls are issued or returns initiated, serialized tracking allows exact units to be identified and isolated. Rather than pulling an entire batch, only the affected serials can be flagged-minimizing disruption and cost. Returned items can also be validated automatically by serial number, ensuring the returned product matches what was originally sold.
- Supports reverse logistics without guesswork
- Enables targeted quarantining and root-cause analysis
- Automates recall documentation for regulators
Example:
A premium wine brand discovers a cork contamination issue in one of its bottling runs. Since each bottle is serialized and linked to its case and pallet, Hopstack allows the fulfillment team to isolate the exact affected serials—down to the specific distributors and retail stores they were sent to. Instead of recalling thousands of cases, they surgically recall only a few hundred, avoiding reputational damage and major financial loss.
Warranty and Lifecycle Tracking
Serial numbers act as digital fingerprints that persist after fulfillment. When units are sold with warranties or service contracts, serial-level history allows:
- Automated warranty validation (based on shipment or manufacturing date)
- Service records tied to individual units
- Support teams to diagnose issues based on serialized configurations or component history
This is especially critical in industries like consumer electronics, industrial tools, or medical devices-where post-sale support and compliance reporting are mandatory.
Example:
A global brand selling smart home security cameras enables unit-level warranty and service tracking through serialization. When a customer calls support with a malfunctioning unit, the team pulls up the full lifecycle of that specific camera—manufacture date, firmware version at shipment, prior warranty claims, and known batch-level issues. This enables faster resolution, reduced fraud, and precise product feedback loops for engineering.
Enhanced Inventory Accuracy
Serialized tracking enforces inventory integrity at the unit level. Each movement-whether a putaway, cycle count, or shipment-is validated against a known serial. This reduces:
- Mis-picks or accidental duplication
- Ghost inventory resulting from scan errors
- Internal shrinkage or process-related loss
Operators can no longer simply scan a SKU and move “one of them”-they must scan the specific unit intended, and the WMS enforces this logic throughout the workflow.
Example:
A luxury jewelry 3PL handles serialized SKUs like engagement rings and custom bracelets. With Hopstack, every item must be scanned by serial during receiving, putaway, cycle counts, and shipping. This prevents mix-ups between visually similar SKUs and eliminates the risk of “ghost inventory” from scanning errors. Shrinkage is reduced dramatically, and insurance audits become faster and more transparent.
Audit Readiness and Compliance (FDA, CPSC, TTB, etc.)
Serialized data captured in a WMS forms a complete digital audit trail. For regulated industries, this provides:
- Immediate response to regulatory inquiries (e.g., “Show all units in Lot X shipped to Region Y”)
- Automated compliance documentation (UDI, DSCSA, TTB batch lineage)
- Structured historical records aligned with retention requirements
Audits, inspections, or certifications are significantly simplified when serial-level histories are already embedded into normal WMS operations.
Example:
An auto parts distributor supplying critical braking components to OEMs must maintain full traceability under ISO/TS and DOT standards. When a safety issue is reported, the brand uses Hopstack to generate an audit trail of all ABS sensors in a specific lot—when they were received, stored, packed, shipped, and to which assembly plants. The report is delivered to regulators within hours, avoiding production delays or regulatory penalties.
Deterring Counterfeits and Theft in Transit
In high-value or fraud-prone industries, serialized tracking deters gray market diversion, counterfeiting, and internal theft. By validating each serial at the point of shipment and receipt:
- Unauthorized substitutions can be flagged in real-time
- Diversion of legitimate units to unauthorized sales channels can be traced
- Supply chain partners can be held accountable via serial-level accountability
Serialization isn't just a traceability tool-it's a trust enforcement mechanism embedded into the fulfillment process.
Example:
A shipment of high-value insulin pumps moves through multiple 3PL hubs before reaching hospitals. Hopstack’s WMS ensures each pump’s serial is scanned and verified at every transfer point. At the final delivery site, any mismatch between expected and received serials flags an alert, preventing the introduction of counterfeit units and helping manufacturers hold logistics partners accountable for loss or tampering.
How a WMS Supports Serialized Inventory
Serialized inventory tracking is only as effective as the system enforcing it-and in high-throughput fulfillment environments, that responsibility falls squarely on the warehouse management system (WMS). A modern WMS does more than record serial numbers; it operationalizes serialization at every step, enforcing integrity, enabling real-time validation, and integrating with upstream and downstream systems for seamless visibility.
Receiving Serialized Goods
Whether serial numbers are supplier-assigned or generated in-house, the WMS must capture and validate them at the point of receipt. This applies to both item-level and multi-level serialized inventory, where the relationships between units, cases, and pallets must be accurately recorded.
This process is often executed via mobile RF or handheld scanning devices and includes:
- Validation against ASN or PO records to ensure that the expected serials—across all levels—were delivered. For multi-level shipments, the system must validate both parent (e.g., pallet or case) and child (unit) serials and their logical linkage.
- Automatic association of serials to lots, SKUs, and inbound shipments, including nesting relationships where applicable (e.g., unit → case → pallet). This structured data forms the basis for downstream traceability.
- Flagging of duplicate, missing, or invalid serials, whether at the unit level or higher packaging levels. This ensures that any discrepancies are caught at the point of receipt, before they impact inventory or fulfillment workflows.
- Optional serialization-on-receipt, where serials are generated and assigned in real time for untagged goods. This is especially useful for product categories like jewelry or auto parts, where incoming items may not arrive with pre-assigned serials.
For example, in a consumer electronics warehouse, a shipment of serialized motherboards may arrive in cases linked to a pallet. If the scanned serials do not match the expected serials in the PO—either at the unit or case level—the system can halt receiving, trigger exception workflows, and notify procurement or quality control teams. This ensures accurate traceability is established from the very first touchpoint.
Serial Tracking During Putaway, Picking, and Packing
Serialized control must persist across warehouse workflows-not just at the dock.
- Putaway: The WMS maps each serial to a location ID, enabling unit-level traceability. Directed putaway logic may be influenced by serial attributes (e.g., firmware version, lot expiration).
- Picking: Operators are prompted to scan specific serial numbers. This eliminates mis-picks and ensures that serialized orders are fulfilled with the exact units allocated by the system.
- Packing: At packout, serials are verified against the order manifest. Multi-level packaging hierarchies (e.g., unit-to-case, case-to-pallet) are established here, with parent-child relationships recorded and stored by the WMS.
This serialized enforcement is especially important when multiple items in an order may look identical (e.g., same model SKU) but differ by revision, configuration, or batch.
Scanning and Real-Time Validation
The WMS enforces serialization through real-time scan validation, leveraging embedded business rules and system lookups to:
- Confirm that serials are valid and active
- Prevent duplicate shipments or re-scanning of the same serial
- Validate serials against order lines, quarantine lists, or blocklists
- Trigger alerts if unauthorized serials are picked or packed
In environments like medical devices or secure locking systems, this kind of validation prevents regulatory breaches and protects against counterfeit distribution.
Real-Time Inventory Visibility
Every serialized unit becomes its own entity in the WMS-complete with location, status, and transaction history. This enables:
- Unit-level inventory inquiries ("Where is serial #A12345 right now?")
- Trace-back and trace-forward capabilities for recalls or audits
- Segmentation by serial attributes (e.g., firmware version, manufacture date, certification)
Unlike SKU-based inventory, where quantities are abstracted, serialized inventory in a WMS offers full transparency into what’s where-and why.

Integration with ERP, Compliance Systems, and POS
Serialization workflows don’t stop inside the warehouse. A WMS must synchronize serialized data with other systems in real time:
- ERP: For cost tracking, asset management, and post-sale service
- Compliance platforms: For regulatory reporting (e.g., DSCSA, TTB, UDI)
- POS or eCommerce platforms: For warranty activation or return validation
- MES or production systems: To receive serialized component histories for final assembly tracking
These integrations ensure that serial numbers remain consistent across system boundaries-avoiding mismatches, duplicate records, and audit failures.
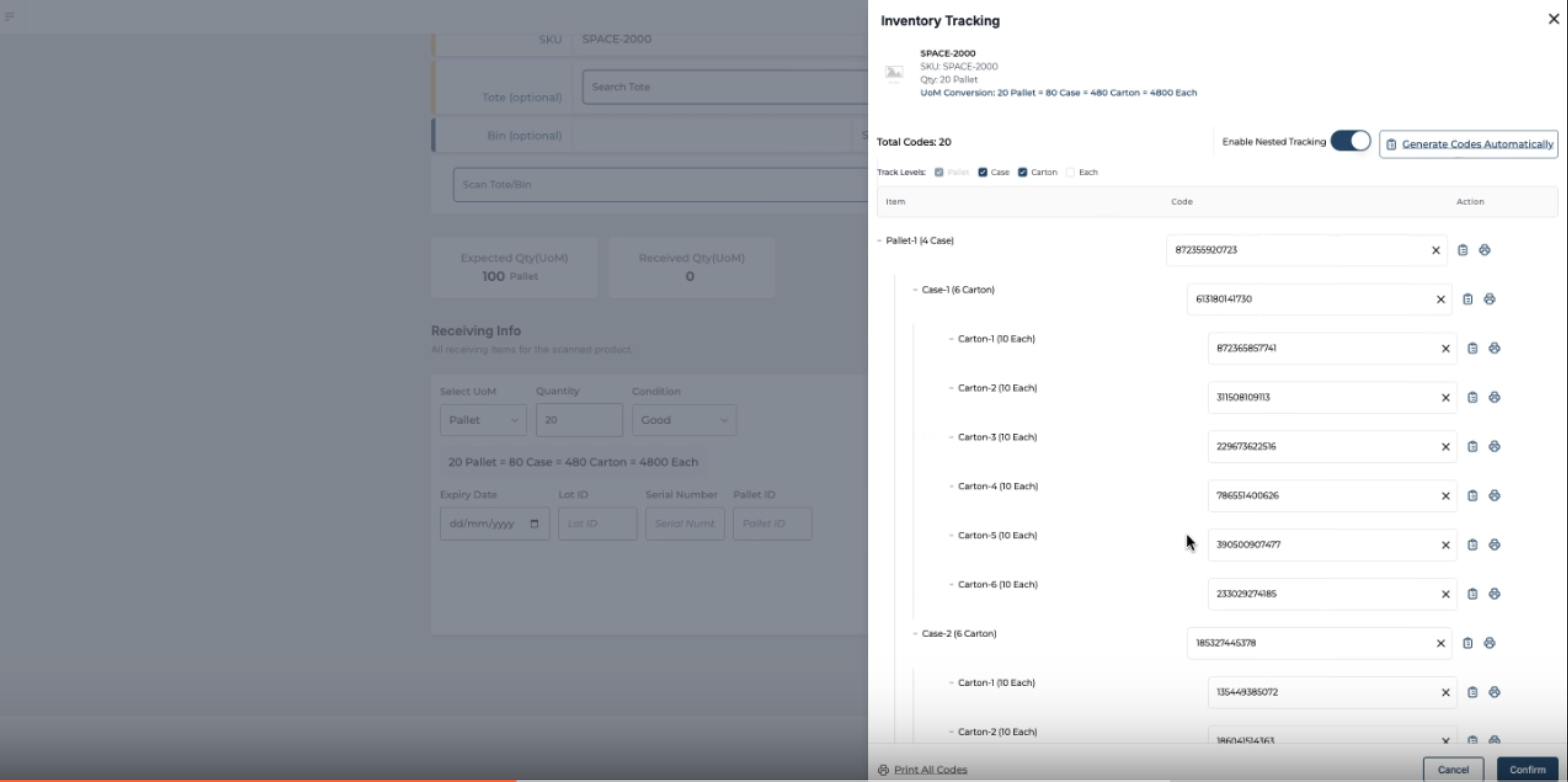
How Multi-Level Serialization Works in Practice
Multi-level serialization-often referred to as nested serialization-goes beyond assigning serial numbers to individual units. It involves establishing and maintaining logical parent-child relationships between serials across packaging hierarchies: from the pallet, to the case, down to the unit. These relationships must be digitally created, stored, and validated in real time by the WMS.
This structure is essential for industries that demand complete traceability across complex distribution chains, component-level recall readiness, or strict chain-of-custody control.
The Hierarchical Model: Pallet → Case → Unit
In a nested serialization setup:
- A pallet is assigned its own serial number (e.g., PLT-0932456)
- Each case stacked on that pallet is also serialized (e.g., CASE-8392, CASE-8393)
- Each unit inside those cases has its own unique serial (e.g., SN-0001 to SN-0012)
The WMS creates a digital map that links each unit serial to its case serial, and each case serial to its pallet. These relationships are maintained throughout all warehouse transactions-including putaway, picking, transfer, and shipping.
This nested structure enables batch recall by pallet, case inspection with drill-down to units, or unit-level service lookup with upward traceability to its original shipment or batch.
Creating Relationships During Receiving (Not Packing)
In most multi-level serialization workflows, parent-child relationships between serialized units, cases, and pallets are established at the time of receiving—not packing. This ensures that traceability is maintained from the very first point of inventory entry and continues across all subsequent warehouse processes.
Depending on how the goods arrive, operators follow one of two workflows:
- If serial labels are already applied to items, cases, and pallets by the supplier:
Operators can begin by scanning the pallet serial, then the case serials under it, and finally the individual item serials inside each case. The system validates each layer and records the hierarchy. - If serial numbers need to be manually scanned or entered:
Operators scan the item serial, then scan it into a case serial. Once all items are linked to their respective cases, the case serials are then scanned into a pallet serial. This establishes the full nested structure.
Once captured, this hierarchy is saved, time-stamped, and stored in the system as a traceable packaging structure. The relationships—unit → case → pallet—are preserved throughout inventory management and become foundational for outbound picking, shipping, recalls, and compliance events.
Automated prompts and real-time barcode validation help ensure no unit is omitted, duplicated, or misassigned. If an operator attempts to scan an item serial that’s already linked elsewhere (e.g., “Serial SN-0011 already assigned to another case”), the system flags the error immediately—maintaining integrity across the serialized hierarchy.
A Real-World Scenario: Consumer Electronics Warehouse
Consider a warehouse shipping smartphones, each serialized at the unit level. These are packed into serialized cartons (10 units per carton), and cartons are stacked on serialized pallets (50 cartons per pallet):
- A specific phone (SN-XL4357) is returned due to a fault.
- The WMS instantly traces it to:
→ Case: CARTON-2024A17
→ Pallet: PALLET-EMEA-9823
→ Shipment: SO#11897, shipped via DHL from DC3 on March 14 - It’s discovered that all phones in that carton were flashed with a faulty firmware version.
- The WMS is used to flag all related serials (9 others) and mark the parent pallet as potentially impacted.
- Only those specific units are quarantined or recalled-not the entire batch or shipment-preserving operational efficiency while meeting compliance obligations.
System Dependencies and Enforcement
To support this model, a WMS must:
- Maintain parent-child relationships natively, not through custom scripting or bolt-on logic
- Allow dynamic re-nesting, in case a unit is unpacked or re-assigned
- Expose the full serial hierarchy in transaction logs, reports, and external API feeds
- Enforce validation at each packaging level to prevent nesting errors or skipped steps
The moment a serialized unit is placed in the wrong case-or a case is moved without updating the pallet record-data integrity is compromised. The WMS ensures this never happens by enforcing workflow discipline through scan prompts, exception alerts, and automated system checks.
Serialized Inventory Tracking: Implementation Considerations
Choosing the Right Level of Serialization
Not all products require unit-level serialization, and not all workflows benefit equally from nested tracking. The WMS must support flexible serialization strategies based on SKU attributes, regulatory needs, or customer requirements.
Unit-level only is ideal for high-value items or those requiring warranty tracking, such as smartphones and jewelry. Case-level only works well for high-volume but less sensitive SKUs, such as consumer packaged goods. Full pallet-case-unit nesting is critical where complete traceability and packaging lineage are necessary, such as in the medical device or electronics industries.
Defining a clear serialization policy ensures a balance between traceability and operational complexity, helping avoid unnecessary overhead or inefficiency.
Preparing Your Team and Warehouse Processes
Serialization introduces stricter controls and more touchpoints, making training and process updates essential. Operators can no longer bulk-receive or blind-pick items-each serial must be captured, verified, and matched against system expectations.
This means warehouse operators must scan every serial during receiving, picking, and packing. Supervisors will need to be equipped with tools to audit serialized hierarchies, reassign serials, or troubleshoot exceptions when serial numbers are misread, missing, or misassigned.
Cycle counters and quality assurance teams also need to understand how serialized inventory behaves differently from non-serialized goods. This might mean adjusting workflows to allow for detailed scanning or even reconfiguring packing stations with fixed-mount or hands-free devices to minimize friction.
Labeling, Scanning, and Hardware Requirements
Successful serialization workflows depend on durable, scannable barcodes-usually QR codes or 2D formats-that map accurately to digital serial records. Label placement is essential; labels should be placed in easily visible locations on the product or packaging to ensure smooth scanning without requiring unpacking.
Additionally, if labels are generated internally, printers must be capable of dynamically outputting serialized labels based on real-time WMS data. Hardware selection is also a key consideration. Scanners must be able to handle the required range, speed, and scanning density, particularly in high-throughput or complex environments like palletized outbound lanes where multi-level nesting occurs.
System Integration and Data Synchronization
Serialization does not operate in a silo. To be effective, serialized data must be integrated seamlessly with other systems. Serial numbers must be transmitted into upstream ERP systems for financial reconciliation and compliance, and downstream to customer-facing systems for returns, warranty validation, or regulatory reporting.
Serialization often intersects with complex compliance requirements, such as the FDA’s UDI system for medical devices or the Drug Supply Chain Security Act (DSCSA) for pharmaceuticals. The WMS needs to natively support these outputs, or middleware layers and APIs will be necessary for smooth data exchange between systems.
Avoiding Common Mistakes
Even with the right tools in place, mistakes in serialized inventory can disrupt operations. Over-serializing low-risk or low-value items adds unnecessary scanning overhead, while relying on manual entry for serial numbers introduces the risk of errors or misassignments. It’s also critical to enforce strict validation during the picking, packing, and shipping processes to ensure that serialized inventory is tracked accurately at every stage.
Systems should be thoroughly tested in sandbox environments before going live to verify that the nested relationships and packaging hierarchies are correctly implemented. Additionally, exception handling workflows should be developed for damaged labels, mis-scanned serials, or issues with split shipments to maintain data integrity.
Industries That Can’t Afford to Skip Serialization
Wine & Spirits: Regulatory Compliance and Authenticity
The wine and spirits industry operates under stringent traceability regulations, particularly regarding excise tax compliance, authenticity, and anti-diversion controls. In the U.S., the Alcohol and Tobacco Tax and Trade Bureau (TTB) mandates detailed recordkeeping and traceability throughout the production and distribution process, especially for imported goods and bonded operations. Labels, serial lot tracking, and audit trails are central to ensuring compliance (TTB.gov).
In the European Union, excise goods such as wine and spirits are regulated under the Union Customs Code and Excise Movement and Control System (EMCS), which require digital tracking of duty-suspended movements across borders (European Commission). Serialization helps producers and distributors maintain a chain of custody down to the bottle level—supporting anti-counterfeiting measures, preventing gray market diversion, and enabling real-time verification during audits.
By uniquely identifying bottles or cases, serialization strengthens transparency and traceability—ensuring legal compliance and protecting brand equity.
Jewelry: Preventing High-Value Theft and Enhancing Security
High-value items like diamond rings, luxury watches, or gold bars are especially vulnerable to theft, fraud, and unauthorized sales. Assigning a unique serial number to each piece creates a permanent record of ownership and movement—from manufacturing to sale and even customer returns.
Serialized tracking adds a critical layer of security: stolen or tampered items can be traced, suspicious returns identified, and internal shrinkage dramatically reduced.
Since traceable items are harder to sell on illicit channels, serialization also inhibits black‑market resale and protects both insurers and retailers in loss investigations
Medical Devices: Ensuring Patient Safety and Compliance
In the U.S., the FDA’s UDI (Unique Device Identification) system requires most medical devices to carry a unique identifier on their labels and packaging, including a device identifier (DI) and, where applicable, a production identifier (PI) such as serial number or lot code.
This data must also be submitted to the FDA’s Global Unique Device Identification Database (GUDID). In the EU, Regulation (EU) 2017/745 mandates UDI marking and registration in the European database EUDAMED, providing traceability from production through post‑market surveillance and aiding recall processes.
By serializing each device, manufacturers and distributors can quickly identify and quarantine affected units, support safety investigations, validate warranty claims, and satisfy regulatory inspections—all while safeguarding patient health and maintaining compliance.
Consumer Electronics: Managing Component-Level Traceability
Serialization is increasingly essential in consumer electronics—especially for ensuring traceability of individual components through the supply chain and product lifecycle.
Major traceability frameworks like IPC‑1782 outline mandatory data collection requirements for electronic components, including manufacturer, lot code, and date code, ensuring each part can be traced back to its origin and production batch.
This form of serialization supports:
- Quality control & warranty resolution, enabling manufacturers to trace field failures back to specific batches or suppliers.
- Targeted recalls, where only units built with faulty components need to be isolated—not entire production runs.
- Anti-counterfeiting and supply chain integrity, especially when integrated with standards such as SAE AS6496, which restricts the distribution of semiconductors outside the authorized supply chain
Regulatory drivers further reinforce this:
- In the EU, the upcoming Digital Product Passport (DPP) under the circular economy framework will require detailed product origin and composition records for electronics by 2026.
- Environmental legislation like RoHS (2011/65/EU) and REACH demands verification of material composition for component-level sourcing—a task made feasible only through serialized tracking of parts and supplier declarations
Thus, serialized component tracking is fast becoming non-negotiable—not just for operational efficiency, but also for compliance, environmental responsibility, and brand protection across electronics and IoT device supply chains.
Serialized Locks: Enhancing Security & Access Control Systems
Note: No verifiable public regulation mandates serialization by sector, so we maintain a qualitative illustration aligned with best practices.
In high-security environments—such as commercial buildings, governmental infrastructure, or critical installations—each physical access device (locks and keys) benefits from being uniquely serialized and recorded. This enables:
- Precise tracking of distribution, usage, and maintenance over time.
- Immediate detection of unauthorized duplication or substitution, ensuring that devices with inconsistent serials cannot be activated or installed.
- Audit-friendly traceability, allowing security teams to prove control over who accessed what, when—permitting seamless compliance with internal or external audits.
While formal regulations may not require serialized locks specifically, robust serialization is tacitly expected in sectors with strict security standards like finance, defense, and national infrastructure. Let me know if you’d like to explore compliance frameworks or standards that reference hardware tracking in this context.
Pharmaceuticals: Combating Counterfeit Drugs and Ensuring Compliance
The pharmaceutical sector is the most strictly regulated when it comes to serialization, largely due to patient safety risks and the global rise in counterfeit drugs. Two cornerstone regulations mandate serialization:
- U.S. Drug Supply Chain Security Act (DSCSA) – Requires prescription drugs to be marked with a unique product identifier (GTIN, serial number, lot number, and expiration date) at the package level. As of November 2024, interoperable, electronic, unit-level traceability across the supply chain will be mandatory (FDA.gov).
- EU Falsified Medicines Directive (Directive 2011/62/EU) – Requires serialization and tamper-evident packaging for all prescription medicines sold in Europe. Each product must be uploaded to the EU’s central verification system (EMVS) for authentication before dispensing (ec.europa.eu).
Serialization ensures that:
- Every unit is uniquely identifiable, enabling complete traceability from manufacturer to dispenser.
- Suspicious or diverted products are flagged during verification, helping remove illegitimate items from the supply chain.
- Regulatory audits, recalls, and returns can be managed with precision, reducing exposure for manufacturers and ensuring compliance.
Additionally, countries like India (via iVEDA), Brazil (SNCM), and Russia (Chestny ZNAK) have implemented or are implementing mandatory serialization frameworks for domestic and export drug markets—making serialized tracking a global requirement rather than a regional one.
Choosing the Right WMS for Serialized Inventory
When selecting a Warehouse Management System (WMS) for serialized inventory, it is essential to choose a system that offers a set of features designed to streamline serialized tracking and ensure operational efficiency. Below are the key features and capabilities to consider:
1. Real-Time Serial Tracking Across All Stages
A robust WMS must be capable of tracking serial numbers in real-time across receiving, picking, packing, and shipping. This ensures that every serialized item is logged and traced throughout the warehouse process, providing end-to-end visibility and reducing errors.
2. Multi-Level and Nested Serialization Support
For companies handling products that require multiple layers of serialization—such as pallets, cases, and units—your WMS should support multi-level and nested serialization. This capability is crucial for industries that require hierarchical tracking, such as medical devices, consumer electronics, and pharmaceuticals, as it ensures complete traceability across all packaging levels.
3. Seamless Integration with ERP and Compliance Systems
The WMS must integrate seamlessly with other systems in your supply chain, such as ERP software, shipping tools, and compliance systems. This integration ensures that serialized data flows consistently and accurately between systems, helping to streamline operations and maintain compliance with regulatory requirements.
4. Advanced Reporting and Audit Capabilities
Serialized inventory management requires transparency and accountability. A good WMS will provide advanced reporting features that enable users to track inventory movements, perform audits, and quickly retrieve serialized data for recall or compliance purposes. The system should also provide detailed insights into inventory levels, allowing businesses to monitor product flow and adjust operations as needed.
5. User-Friendly Interface and Mobile Support
To minimize human error and ensure operational efficiency, the WMS should have a user-friendly interface. The system should also support mobile devices for barcode scanning, which is essential for warehouse staff who need to quickly capture serial numbers during high-volume operations. This mobile support improves accuracy and speeds up the process of managing serialized inventory.
6. Scalability and Flexibility for Growing Operations
As your business grows, so do your serialized inventory needs. The WMS should be flexible enough to scale with your operations and adaptable to future requirements. It should allow for easy upgrades, integration with additional systems, and the ability to support larger volumes of serialized products without compromising performance.
Hopstack offers a comprehensive WMS solution that provides all of the above features. With its seamless integration capabilities, real-time inventory visibility, support for multi-level serialization, and flexible scalability, Hopstack ensures that your business can effectively manage serialized inventory while staying compliant with industry regulations.
Conclusion
Serialized inventory tracking is no longer just a trend; it’s a necessity for industries where traceability, compliance, and security are paramount. Whether you're in pharmaceuticals, medical devices, electronics, or high-value goods, implementing a WMS capable of handling serialized inventory is a critical investment in operational efficiency and regulatory adherence.
By leveraging advanced features like multi-level serialization, seamless system integration, and real-time tracking, businesses can stay ahead of the curve, reduce risks, and improve customer satisfaction. With solutions like Hopstack, companies can simplify complex inventory workflows while ensuring they meet compliance standards and deliver on quality expectations.
FAQs
What industries need serialized inventory tracking?
Industries such as pharmaceuticals, medical devices, consumer electronics, wine & spirits, jewelry, and serialized locks rely heavily on serialized inventory tracking for regulatory compliance, fraud prevention, and detailed product traceability.
What is the difference between item-level and multi-level serialization?
Item-level serialization refers to assigning a unique serial number to each individual product, while multi-level serialization includes the ability to track serial numbers across different packaging layers—such as pallet, case, and unit—offering deeper traceability within a supply chain.
What are the benefits of nested serialization in inventory management?
Nested serialization allows for hierarchical tracking, linking serial numbers across multiple packaging layers (e.g., from a pallet down to a single unit). This ensures full traceability of a product’s journey through the supply chain, aiding in recall management, inventory accuracy, and compliance with regulatory standards.
Can serialized inventory tracking help with product recalls?
Yes, serialized inventory tracking provides full visibility into the history of a product, making it easy to identify and isolate faulty or defective products quickly during a recall, reducing potential harm to customers and minimizing business risks.
What features should I look for in serialized inventory software?
Key features include real-time serial tracking, multi-level and nested serialization support, system integration capabilities, advanced reporting and audit features, user-friendly interfaces, and mobile support for scanning.
Is serialization required for regulatory compliance in certain industries?
Yes, industries like pharmaceuticals, medical devices, and alcohol are legally required to serialize products for traceability, fraud prevention, and compliance with laws such as the FDA’s Unique Device Identification (UDI) system or the Drug Supply Chain Security Act (DSCSA).

%20(1).png)

.png)
